
Biosimilars Market Size and Forecast, By Indication (Blood Disorders, Oncology, Chronic & Autoimmune Diseases), And Trend Analysis, 2014 - 2024
- Published: August, 2017
- Format: Electronic (PDF)
- Number of pages: 89
- Industry: Healthcare
Industry Insights
The global biosimilars market size was valued at 3.39 billion in 2016 and is expected to grow on account of increasing prevalence of chronic diseases such as cancer and autoimmune disorders. Additionally, increasing prevalence of arthritic disorder and obesity across the globe in geriatric population is anticipated to drive the demand for biosimilar over the study period.
Global biosimilars market revenue, 2014 - 2024 (USD Billion)
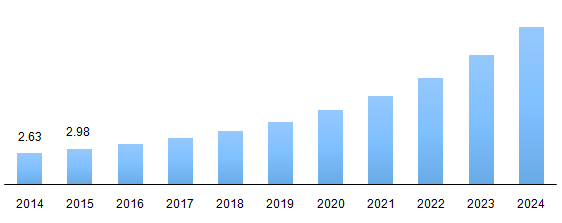
The industry for biosimilars is expected to show lucrative growth after 2018 due to the expiry of various patents during the period. For instance, a patent for Adalimumab by the brand name Humira is expiring in 2018. Additionally, biosimilars are cost effective compared to their counterpart (biologics) which in turn is expected to boost the industry over the forecast period. Globally, increasing cost of cancer treatment is placing a noticeable burden on healthcare systems, largely a result of costly biologics, where an increasing inclination towards biosimilars is being observed.
The global market is expected to observe changes owing to increasing number of approvals of biosimilars across the globe. For instance, in March 2015, Zarxio (Biosimilar to Neupogen) became the first biosimilar to be approved in the U.S. industry. Additionally, in April 2016, Inflectra (Biosimilar to Remicade) was approved in the U.S. for the treatment of Crohn’s disease.
Besides an increasing number of biosimilars under the pipeline, the global market is expected to witness increasing investment on R&D. For instance, there are more than 50 biosimilars to 18 reference molecules in the FDA’s Biosimilar Development Program. However, highly regulated market and fewer success rates are expected to hamper the growth.
Segmentation by Indication
• Blood Disorders
• Oncology
• Chronic & Autoimmune Diseases
• Others
Oncology segment contributed maximum revenue share in 2016 and is expected to maintain its position over the forecast period owing to increasing prevalence of cancer across the globe. Furthermore, high therapy cost of biologics in the treatment of cancer is expected to play an important factor in the increasing inclination towards biosimilars.
Fast approvals from regulatory bodies will flourish the industry. Additionally, in July 2017, Biocon - Mylan’s breast cancer biosimilar got approval from the US FDA’s Oncologic Drugs Advisory Committee. If the drug gets FDA’s nod, Biocon will be the first Indian drug manufacturer to crack this highly regulated industry. The U.S. market for blood disorders is also expected to show significant growth owing to the increasing cases of leukaemia and anaemia.
Segmentation by Region
• North America
• Europe
• Asia Pacific
• Rest of the world
In 2016, Europe dominated the global industry owing to rising number of approvals from European Medicine Agency (EMA). The first biosimilar was approved in 2006, whereas in the U.S. the first biosimilar was approved in 2015. Europe has been the market leader in since its first approval. Rising adoption of biosimilars in Europe is the key factor for growth in the region. However, stringent rules and regulations are expected to restrain the growth of the market in the region.
Asia Pacific is expected to grow at the fastest CAGR over the forecast period. The market in this region is expected to grow owing to increasing R&D by pharmaceutical companies in India and China. Increasing prevalence of cancer and other medical conditions in the region is expected to drive the market in the region. Furthermore, rising geriatric population in countries such as Japan and India is projected to provide a lucrative opportunity for the growth of the market. The pharmaceutical companies are focusing on mergers & acquisitions to expand their portfolio in the global industry. For instance, in February 2017, Aurobindo Pharma Limited completed its acquisition of four cell culture derived biosimilar product from TL Biopharmaceutical AG to enter into the global market.
Competitive Landscape
The global biosimilars market is consolidated in nature with the majority of the share held by a few well-established players. The key players include Pfizer Inc., Teva Pharmaceutical Industries Ltd., Sandoz International GmbH. The manufacturers are concentrating on getting approvals in various regions to increase their presence globally. Other players include Biogen Idec, Inc., Genentech (Roche Group), Celltrion and Biocon.

Choose License Type
- World's largest premium report database
- Transparent pre & post sale customer engagement model
- Unparalleled flexibility in terms of rendering services
- Safe & secure web experience
- 24*5 Research support service
